Experimental therapies research
Principle investigator: Prof. Dr. Andreas Lutterotti
Research focus
Under this core topic (experimental therapies research) we aim to address unmet medical needs in MS with new therapeutic approaches.
The research will involve:
- Conduct of first-in-man (phase I) and proof-of-concept phase II trials with new therapeutic approaches. This will involve all aspects from preclinical safety studies through the regulatory process and on-site trial conduct.
- Early phase trials will be accompanied by ancillary studies, to address mechanisms of action of experimental treatments, to identify biomarkers and to get insight into the pathogenesis of the disease. The respective studies (immunological, imaging, electrophysiological) will be approached in collaboration with partners in the CRPPMS.
- As further aspect of the research we will improve current trial designs, develop stratification schemes and assess new outcome parameters to measure specific aspects of the disease (inflammation, neurodegeneration).
Current trials
Recruiting patients
1) NanoCl iv: Treatment of Relapsing-Remitting Multiple Sclerosis with RNS60 Administered Intravenously.
Trial design: Open-label, baseline-to-treatment, two-center, phase IIa trial.
Primary objective: To investigate the efficacy, safety and tolerability of RNS60 administered intravenously in patients with RRMS.
Investigational product: RNS60 is an electrokinetically altered aqueous fluid. Chemically, RNS60 is composed of saline and oxygen. The processing of RNS60, which involves Taylor-Couette-Poiseuille (TCP) flow in the presence of elevated oxygen levels, is hypothesized to produce charge-stabilized nanostructures (CSN) that exhibit electrical fields. The CSN, by virtue of their size, charge, and stability, are thought to be responsible for the biological effects of RNS60. In cellular and animal studies, RNS60 has demonstrated anti-inflammatory effects by downregulating proinflammatory cytokines (e.g., IL-4, IL1β, IL-8, TNF-α, iNOS) and up-regulating anti-inflammatory cytokines (e.g., IL-10), depending on the disease model tested.
- Choi S et al. 2014, Frontiers in Synaptic Neuroscience 6 : 2.
- Khasnavis S et al. 2012 Journal of Biological Chemistry 287 (35) : 29529-42
- Modi K et al. 2014 PLoS ONE 9 (8) : e103606.
- Mondal S et al. 2012. PloS ONE 7 (12) : e51869.
- Roy A et al. 2014 PloS ONE 9 (7) : e101883.
Recruiting patients
2) ETIMSred IIa: A phase I/IIa trial on safety, tolerability and efficacy of myelin peptide-coupled erythrocytes.
The ETIMSred is a Wyss Translational Center Zürich project and further details can be found at following webpage:
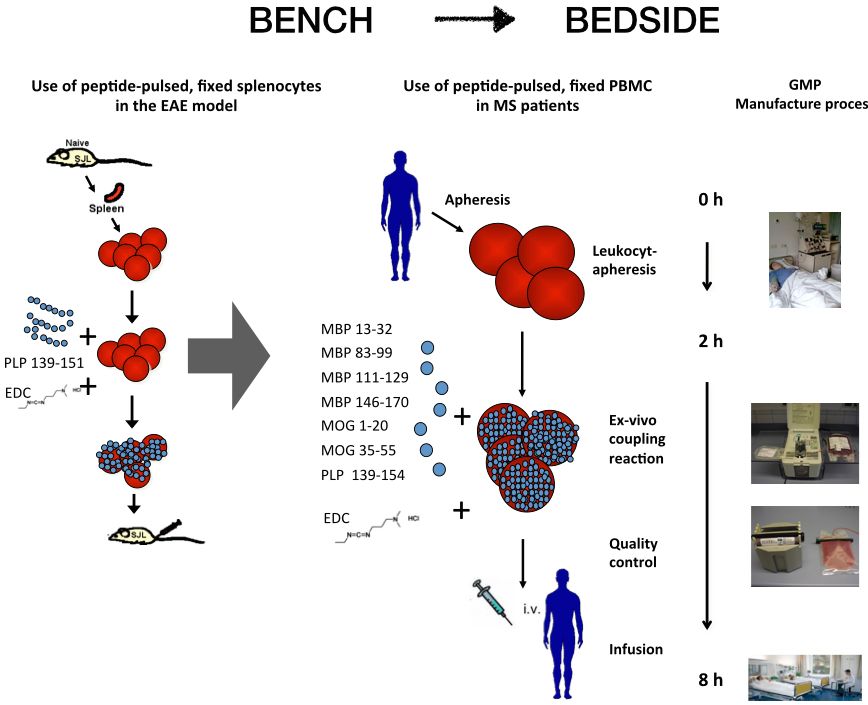
Background: In order to induce immune tolerance in MS patients, we adopted a very promising tolerization strategy that employs autologous peripheral blood mononuclear cells (PBMC) pulsed with myelin peptides and chemically fixed with the cross linker 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) as tolerogenic vaccine. Over the last few years we developed a manufacture process for the ex vivo preparation of antigen-coupled cells and assessed the feasibility, safety and tolerability of a single infusion of peptide coupled PBMC in a first-in-man trial in patients with MS. Our main objective is to advance the treatment strategy further, using antigen-coupled red blood cells (RBCs) as tolerogenic vaccine and to assess its safety, efficacy and mechanisms of action in a phase I/IIa trial in MS patients.
- Lutterotti A et al. 2013 Science Translational Medicine 5, 188ra175
- Peschl P. et al. 2014 Multiple Sclerosis
In preparation
3) Hydroxytyrosol as a treatment of multiple sclerosis
Background: Identified by Puleva Biotech during systematic screens for natural compounds that cross the blood-brain-barrier (BBB) as one that very easily accesses the CNS. Hydroxytyrosol (HT) is contained in first-pass (extra virgen) olive oil, olive leaves and olive waiste water (during production of olive oil) as well as grapes. Among its different biological effects HT is one of the most powerful antoixidants and has a robust anti-inflammatory effect. We aim to assess the safety, tolerability and neuroprotective effects of HT in patients with MS